International Newsletter – August ’23
Dear reader,
Hereby you receive our newsletter, with the following topics:
- PFAS
- ‘’Free’’ inorganic substance in (spore) fertiliser
- Part 1 of the Nutrients series: theme pH, EC and precipitation
- New raw materials in potting soil requires new strategy
PFAS
Although the EU legislation will only take effect from 2025, retail in Germany already applies the so-called guideline values for fruit and
vegetables. That is why trade and growers were already held accountable for exceeding the guideline value with their product. There are 4 types of PFAS guide values.
On 7 February 2023, a European proposal to ban PFAS was published. The ban will apply from 2025 to the production, use, sale and import of more than 10,000 chemicals, which are in thousands of products that we use every day.
As of May 2023, Normec Groen Agro Control, based in Delfgauw, is accredited to analyze PFAS in soil and water, but also in food.
This accreditation for PFAS offers the possibility to monitor PFAS in soil and water in an easy and reliable way. Think of testing irrigation water. This prevents PFAS from being introduced into the soil and finally into the final product.
In addition to testing for PFAS, Normec Groen Agro Control also offers support in setting up monitoring programs, sampling and root cause analyses. In addition, Normec Groen Agro Control is also the first commercial lab in the Netherlands accredited to analyze PFAS in food. If you would like more information about PFAS in the various products, please visit our website; www.agrocontrol.nl. You can also reach us for questions on 015-2572511
‘’Free’’ inorganic substances in (spore) fertiliser
Our lab regularly measures fertilizers for use in potting soils but also for substrate crops. In addition, it has recently been noticed that in ”pure” spore fertilizers other fertilizers and even heavy metals come along. If you want to be sure that you know what you are giving in terms of fertilizer, have it measured.
Ask for Head and Spore elements in % after dissolution in water with soluble fertilizers
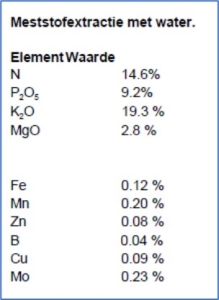
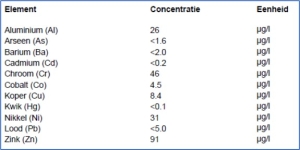
Ask explicitly to measure additional heavy metals, if you are interested in doing so. Here is an example of pure chelate iron with quite a few heavy metals in it such as Aluminum, Chrome and Nickel.
For more information, you can contact our product manager Ines van Marrewijk.
ContactPart 1 of the Nutrients series; theme pH, EC and precipitation
The EC (electrical conductivity) is a measure of the concentration of ions (salts) in water, soil and substrate moisture. The more ions dissolved in the water, the higher the EC.
When analysing the water or substrate, it becomes clear that the measured EC is determined not only by nutritional elements but also by ballast salts (chloride and sodium). In order to make a good comparison with the target value, the measured EC will therefore first have to be converted to the EC (v), the so-called nutritional EC. The EC (v) is obtained by reducing the measured EC by 0.1 x the highest value of sodium or chloride, or standard with sodium in case chloride is used as fertilizer. The EC(v) can then be used to convert the measured nutrient content to the target EC indicated as EC(c).
The EC is not only a control tool for fertilization, but can also be used as a control agent for crop growth. Due to a high EC around the root, you can control the crop generatively, because you increase the osmotic value in the root environment, so that the plant can absorb water less easily, so that the stretching of the cells (vegetative growth) is inhibited. A low EC, on the other hand, facilitates water absorption and can lead to more root pressure, especially with little evaporation. At the same time, with a low EC, you give fewer nutritional elements, which can cause a nutritional deficiency earlier.
The EC in the substrate is not only determined by the settings on the fertilization unit, the growth rate and thus the absorption of the crop also plays an important role. Because in the summer the plant absorbs more and more water in relation to nutrients, a light reduction on the EC is often used during this period, to prevent the EC from increasing. What is often forgotten is that the EC also influences the absorption of individual elements. For example, potassium is better absorbed at a high EC and calcium is worse. This has to do with an increase in competition (antagonism) in absorption between potassium and calcium at a higher concentration, but also because the calcium absorption, unlike potassium, depends on the water absorption by the plant and this is inhibited at a high EC.
The pH is a scale to indicate acidity and determines to a large extent the solubility of the nutrients. Too high a pH can indirectly inhibit crop growth because nutrients are less easily absorbed, while too low a pH can cause problems with root quality. Phosphate and trace elements get out of so
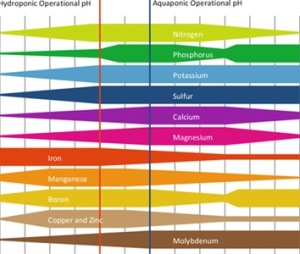
lution at a high pH or can precipitate with other elements. Magnesium washes out faster at a low pH, causing a shortage earlier. For each of the nutrients, a specific pH range applies within which the solubility is optimal, on average for On average for all elements it is between 5.5-6.5.
Normec Groen Agro Control regularly performs analyses on clogged droppers, in which the elements iron, phosphate, zinc and calcium are often found. ”Research on pollution” is combined with organic pollution so that it becomes clear what is most present.
The pH can be controlled with the fertilization unit, often via a separate pH control. This can in any case prevent nutritional elements from precipitating in the drip hoses. If the pH has to be corrected with a lot of acid at the unit, it is advisable to acidify the water beforehand. This makes the control at the unit more stable.
 In the root environment, the pH of the feed water plays a limited role, because the plant itself largely determines the pH around the roots. When cations (K+, Ca2+, Mg2+, etc) are absorbed, acid ions (H+) are excreted by the roots, causing the pH to drop, while when anions (NO3-, PO4-, SO42-) are absorbed H+ so that bicarbonate is formed in the root environment and the pH rises. The balance between the uptake of cations and anions thus determines the pH in the substrate. This balance is not always equal, but can be influenced by the growth phase and growth rate of the crop. More nitrate uptake with rapid growth results in a higher pH and a strong generative development in which more potassium is absorbed gives a lower pH. Finally, bicarbonate in the starting water can also cause the pH in the substrate to rise. A little bicarbonate (up to 0.5 mmol/l) in the drip water ensures that the pH remains more stable and cannot sink at night under the influence of microbiological processes. Our laboratory reports the bicarbonate content based on the total acid buffering effect of the water. This not only looks at the buffer effect of bicarbonate, but also at that of other elements, such as phosphate. This creates a more reliable picture of what the influence of the bicarbonate is on the pH and exactly how much acid is needed to lower the pH to the desired level.
In the root environment, the pH of the feed water plays a limited role, because the plant itself largely determines the pH around the roots. When cations (K+, Ca2+, Mg2+, etc) are absorbed, acid ions (H+) are excreted by the roots, causing the pH to drop, while when anions (NO3-, PO4-, SO42-) are absorbed H+ so that bicarbonate is formed in the root environment and the pH rises. The balance between the uptake of cations and anions thus determines the pH in the substrate. This balance is not always equal, but can be influenced by the growth phase and growth rate of the crop. More nitrate uptake with rapid growth results in a higher pH and a strong generative development in which more potassium is absorbed gives a lower pH. Finally, bicarbonate in the starting water can also cause the pH in the substrate to rise. A little bicarbonate (up to 0.5 mmol/l) in the drip water ensures that the pH remains more stable and cannot sink at night under the influence of microbiological processes. Our laboratory reports the bicarbonate content based on the total acid buffering effect of the water. This not only looks at the buffer effect of bicarbonate, but also at that of other elements, such as phosphate. This creates a more reliable picture of what the influence of the bicarbonate is on the pH and exactly how much acid is needed to lower the pH to the desired level.
New raw materials in potting soil requires new strategy
The composition of a substrate affects the growth of plants. This is because the different raw materials that make up a substrate have specific physical, chemical and biological properties. An adjustment of the composition will therefore have an effect on growth without changing the cultivation strategy. New (renewable) raw materials, such as wood fibre or compost, usually have a lower water retention, which means that the water supply runs out earlier and therefore needs to be irrigated more frequently. At the same time, many renewable raw materials have a lower capacity to bind nutrients (CEC), which means that a larger part of the food washes out during casting and the EC in the pot / mat remains a lot lower. This requires a more regular potting soil analysis and an adapted fertilization strategy.
An important property of peat is that it naturally has a low pH, which is very beneficial for the availability of nutritional elements. Many new raw materials have a high pH, with the risk that a nutrient deficiency occurs more quickly. For example, more ammonium must be given in fertilization.
In the case of non-composted residual flows, such as wood fibre, biological conversion at the beginning of cultivation will create competition for nitrogen between the plant and bacteria, which can cause a nitrogen deficiency. With an extra nitrogen gift, it must be taken into account that it can become available again later in the cultivation. Composted material contains a lot of potassium, which also requires adjustments in the fertilization scheme. In addition to the physical, chemical and biological changes, there is a risk of pollution. Residual flows can be contaminated with unwanted salts and heavy metals. Furthermore, (natural) substances from the new material can have an undesirable effect on growth. Think of substances that can be released from compost or wood, or other fibers. Even pesticide residues occur in it.
Monitoring during cultivation will therefore become even more important. Sample the growing medium more often so that watering and fertilization are accurately adjusted.
Feedback & Questions
Normec Groen Agro Control guarantees reliable analyses and good service. We are open to points for improvement and questions, and therefore would like to get in touch with you. This can be done via the link below to our general contact form or send an email directly to sylvia.toutziari@normecgroup.com or international.agro@normecgroup.com
Contact